Answer: Number of balloons that can be filled with He are 1397.
Step-by-step explanation:
The given data is as follows.
V = 25 L He , P = 131 atm
T =
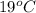 = (19 + 273) K
= (19 + 273) K
= 292 K
According to ideal gas equation,
PV = nRT
where, n =
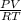
=
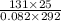
= 136.76 mol of He
The data for small balloons is given as follows.
T =
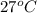 = (27 + 273) K
= (27 + 273) K
= 300 K
P = 732 = 0.963 atm
V = 2.50 L
Now, we will calculate the number of moles as follows.
n =
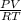
=
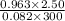
= 0.098 mol
So, number of balloons that can be filled with He are calculated as follows.
n =
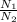
=
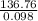
= 1397.60 balloons
or, = 1397 balloons (approx)
Thus, we can conclude that number of balloons that can be filled with He are 1397.