Complete question:
The volume V of a fixed amount of a gas varies directly as the temperature T and inversely as the pressure P . Suppose that V= 42 cm^3 . when T = 84 kelvin and P = 8 kg/cm^2 . Find the volume when T=185 kelvin and P = 10 kg/cm^2
Answer:
The final volume of the gas is 74 cm³
Step-by-step explanation:
Given;
initial volume of the gas, V₁ = 42 cm³
initial temperature of the gas, T₁ = 84 kelvin
initial pressure of the gas, P₁ = 8 kg/cm²
final volume of the gas, V₂ = ?
final temperature of the gas, T₂ = 185 kelvin
final pressure of the gas, P₂ = 10 kg/cm²
From the statement given in the question, we formulate mathematical relationship between Volume, V, Temperature, T, and Pressure, P.
V ∝ T ∝ ¹/p
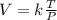
where;
k is constant of proportionality
make k subject of the formula
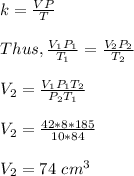
Therefore, the final volume of the gas is 74 cm³