Answer:
the initial polymerization rate
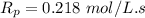
fraction of polymerization rate that is due to free ions = 0.982
fraction of polymerization rate due to contact ion pairs = 0.0176
the average degree of polymerization at the completion of the reaction. =

Step-by-step explanation:
From the table of Polymerization of tetrahydrofuran below; we obtain the following data for Na⁺ counterion
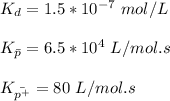
Sodium Naphthalene = 3.2 × 10⁻⁵ M
Concentration of styrene solution = 1.5 M
Now;
![[M^-] = [K_d(M^-C^+])^(1/2)\\ \\\ [M^-] = [(1.5*10^(-7) \ mol/L )(3.20*10^(-5) \ mol/L ) ] ^(1/2)\\\\\ [M^-] = 2.19*10^(-6) \ mol/L](https://img.qammunity.org/2021/formulas/chemistry/college/nfwrfon7trauv2xrq14ju8zbs0pdgiog53.png)
![R_p = K_{\bar{p}} [M^-][M^+] + K_{\bar{p^+}}[M^-C^+][M^-]\\\\R_p = (6.5*10^4 \ L/mol.s)(2.19*10^(-6) \ mol/L)(1.5 \ mol/L)+(80 L/mol.s)(3.2*10^(-5) \ mol/L)(1.5 \ mol/L)\\\\R_p = 0.224 \ mol/ L.s + 0.00384 \ mol/L.s\\\\](https://img.qammunity.org/2021/formulas/chemistry/college/qj55ecs7trsg5bccwzx2h54h5ntg3zn7a6.png)
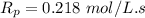
Therefore; the initial polymerization rate
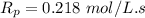
Fraction of polymerization rate due to free ions :
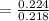
= 0.982
Fraction of polymerization rate due to contact ion pairs .
=
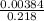
= 0.0176
the average degree of polymerization at the completion of the reaction is :
![\bar{DP_n}= 2 ([M])/([M^- \ C^+])](https://img.qammunity.org/2021/formulas/chemistry/college/ss87cmj9zonfg5quft5v8m3wbi68nhkr95.png)
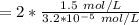
=
