Answer:
The Equilibrium will shift towards the left.
Step-by-step explanation:
The reaction for the formation of nitric oxide is follows,
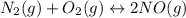
Expression for reaction quotient is as follows,
![Q=([NO]^2)/([N_2][O_2])](https://img.qammunity.org/2021/formulas/chemistry/high-school/6el9l885mk00ehyl2hpgrjaqy2hdkcuje8.png)
Putting the values according to the data given and calculating reaction quotient for the reaction
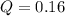
So, the reaction quotient is 0.16.
and the value of K is 0.01 .
Q>K
Since the value of K is less than Q, therefore the reaction will shift towards left.