Answer:
Radioactive decay is defined as the disintegration of an unstable nucleus accompanied by the release of energy.
The different types of radioactive decay are: alpha decay, beta decay, gamma decay; which are accompanied by the release of photons or other subatomic particles.
The mass number (A) and charge number (Z) of the following products of radioactive decay are:
(a) a neutron :
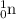
A - 1
Z - 0
(b) an α- particle:
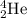
A - 4
Z - (+2)
(c) a positron :
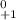
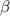
A - 0
Z - (+1)
(d) a photon
A - 0
Z - 0
(e) a β- ray:
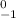
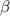
A - 0
Z - (-1)