Answer: +178.3 kJ
Step-by-step explanation:
The chemical equation follows:
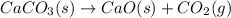
The equation for the enthalpy change of the above reaction is:
![\Delta H^o_(rxn)=[(1* \Delta H^o_f_((CaO(s))))+(1* \Delta H^0f_(CO_2)]-[(1* \Delta H^o_f_((CaCO_3(s))))]](https://img.qammunity.org/2021/formulas/chemistry/college/56zobt333cnnds4rbabdby3br7y3jwzx8l.png)
We are given:
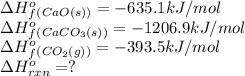
Putting values in above equation, we get:
![\Delta H^o_(rxn)=[(1* (-635.1))+(1* (-393.5))]-[(1* (-1206.9))]](https://img.qammunity.org/2021/formulas/chemistry/college/cmo10kfbd99wjzparzls9h4vilo2gqka4o.png)
The DH°rxn for the decomposition of calcium carbonate to calcium oxide and carbon dioxide is +178.3 kJ