1.95 or 2 is the molarity of a 45.3g sample of KNO3 (101g) dissolved in enough water to make a 0.225L solution.
The correct answer is option b
Step-by-step explanation:
Data given:
mass of KN
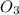 = 45.3 grams
= 45.3 grams
volume = 0.225 litre
molarity =?
atomic mass of KNO3 = 101 grams/mole
molarity is calculated by using the formula:
molarity =
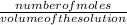
first the number of moles present in the given mass is calculated as:
number of moles =

number of moles =

0.44 moles of KNO3
Putting the values in the equation of molarity:
molarity =

molarity = 1.95
It can be taken as 2.
The molarity of the potassium nitrate solution is 2.