Answer: Free-energy change of a reaction is a measure of the direction in which a net reaction occurs.
Step-by-step explanation:
It is known that free energy change is represented by the symbol
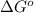 . Free-energy helps in determining the direction of a chemical reaction like if it is taking place in forward or backward reaction.
. Free-energy helps in determining the direction of a chemical reaction like if it is taking place in forward or backward reaction.
For a spontaneous reaction, the standard free-energy change is negative in nature and the reaction will proceed in forward direction.
Thus, we can conclude that free-energy change of a reaction is a measure of the direction in which a net reaction occurs.