Answer:
The ratio of v₂/v₁ is 2
Step-by-step explanation:
Here we have Charles law which can be presented as
P₁/T₁ = P₂/T₂
Therefore,
P₂ = T₂ × P₁/T₁
Also from the kinetic theory of gases we have;
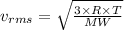
Where:
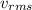 = Rms speed
= Rms speed
R = Universal gas constant
T = Temperature in Kelvin
MW = Molecular weight
We therefore have for the before and after speeds as
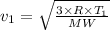 and
and
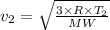
Therefore,
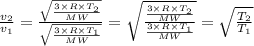
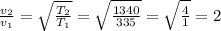
∴ v₂/v₁ = 2.