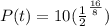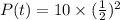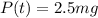Answer:
2.5 mg
Explanation:
We are given that
A=Initial amount=10 mg
Half life,h=8 days
We have to find the mass of radioactive isotope remains after 16 days.
t=16 days
We know that the amount of radioactive isotope after t days is given by
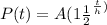
Using the formula