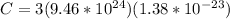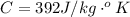Answer:
The specific heat of copper is
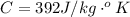
Step-by-step explanation:
From the question we are told that
The amount of energy contributed by each oscillating lattice site is
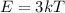
The atomic mass of copper is
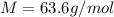
The atomic mass of aluminum is
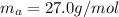
The specific heat of aluminum is
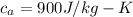
The objective of this solution is to obtain the specific heat of copper
Now specific heat can be defined as the heat required to raise the temperature of 1 kg of a substance by
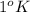
The general equation for specific heat is
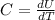
Where
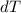 is the change in temperature
is the change in temperature
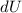 is the change in internal energy
is the change in internal energy
The internal energy is mathematically evaluated as
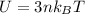
Where
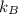 is the Boltzmann constant with a value of
is the Boltzmann constant with a value of
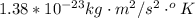
T is the room temperature
n is the number of atoms in a substance
Generally number of atoms in mass of an element can be obtained using the mathematical operation
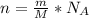
Where
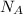 is the Avogadro's number with a constant value of
is the Avogadro's number with a constant value of
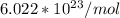
M is the atomic mass of the element
m actual mass of the element
So the number of atoms in 1 kg of copper is evaluated as
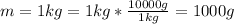
The number of atom is
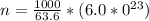
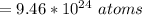
Now substituting the equation for internal energy into the equation for specific heat
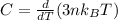
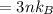
Substituting values