Answer:
0.0812 grams of nitrate ions are there in the final solution.
Step-by-step explanation:
Mass of cobalt (II) nitrate = 3.00 g
Moles of cobalt(II) nitrate =

Volume of the solution = 100 mL = 0.100 L
1 mL = 0.001 L
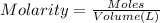
Molarity of the solution =
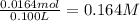
Cobalt (II) nitrate in its aqueous solution gives 1 mole of cobalt(II) ion and 2 moles of nitrate ions.
![[NO_3^(-)]=2* [Co(NO_3)_2]=2* 0.164 M=0.328 M](https://img.qammunity.org/2021/formulas/chemistry/high-school/ajlp9bd3wb7h69k0e3ml6pyy905jjxd5e3.png)
Molarity of the nitrate ion before solution =
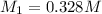
Volume of the nitrate ion before solution =
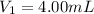
Molarity of the nitrate ion after solution =
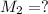
Volume of the nitrate ion after solution =
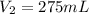
 ( Dilution)
( Dilution)
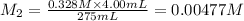
Moles of nitrate ions in 275 ml = n
Molarity of the nitrate ion after solution =0.00477 M
volume of the final solution = 275 mL = 0.275 L
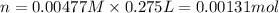
Mass of 0.00131 moles of nitrate ions:
0.00131 mol × 62 g/mol = 0.0812 g
0.0812 grams of nitrate ions are there in the final solution.