Answer: The pH of given solution is 3.74.
Step-by-step explanation:
The given data is as follows.
Mass of lactic acid = 8.52 g, Formula weight of lactic acid = 90.08 g/mol
So, number of moles of lactic acid will be calculated as follows.
No. of moles =
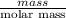
=
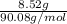
= 0.094 moles
Mass of sodium lactate = 7.93 g, Formula weight of sodium lactate = 112.06 g/mol
Hence, number of moles of sodium lactate is as follows.
No. of moles =
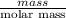
=
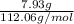
= 0.071 moles
As we know that relation between
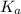 and
and
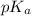 is as follows.
is as follows.
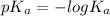
= -log(0.000137)
= 3.86
Using Henderson equation, we will calculate the pH as follows.
pH =
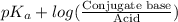
pH =
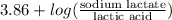
=
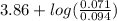
= 3.86 + log (0.755)
= 3.86 - 0.121
= 3.74
Therefore, we can conclude that pH of given solution is 3.74.