Answer:
Weight % of NH₃ in the aqueous waste = 2.001 %
Step-by-step explanation:
The chemical equation for the reaction

Moles of HCl = Molarity × Volume
= 0.1039 × 31.89 mL ×
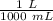
= 0.0033 mole
Total mass of original sample = 25.888 g + 73.464 g
= 99.352 g
Total HCl taken for assay =
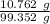
= 0.1083 g
Moles of NH₃ =
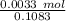
= 0.03047 moles
Mass of NH₃ = number of moles × molar mass
Mass of NH₃ = 0.03047 moles × 17 g
Mass of NH₃ = 0.51799
Weight % of NH₃ =
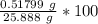 %
%
Weight % of NH₃ in the aqueous waste = 2.001 %