The theoretical mass of ammonia is 56.6 grams when reaction is begun with 10.00 grams of each reactant.
Step-by-step explanation:
Data given:
mass of reactants = 10 gram
The reactants are hydrogen and nitrogen gas.
Theoretical mass of ammonia =?
balanced chemical reaction is :
3
 +
+
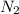 ⇒ 2 N
⇒ 2 N
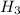
Atomic mass of hydrogen gas = 2 grams/mole
atomic mass of nitrogen gas = 28 grams/mole
number of moles of each reactant is determined:
number of moles =

putting the values for hydrogen gas
number of moles =
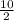
number of moles = 5
number of moles of nitrogen gas =
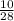
= 0.35 moles
from the balanced equation,
3 moles of hydrogen gas reacted to give 2 moles of ammonia
5 moles of hydrogen will give x moles
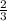 =
=
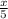
3x =10
x = 3.33 moles
mass of ammonia produced = number of moles x atomic mass
= 3.33 x 17
= 56.61 grams of ammonia produced from hydrogen
For nitrogen gas,
1 mole nitrogen gas reacted to give 2 moles of ammonia gas
0.35 moles will give x moles
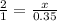
x = 0.7 moles of ammonia formed
mass = number of moles x atomic mass
= 0.7 x 17
= 11.9 grams
Thus the limiting reagent is hydrogen gas in the reaction.
The mass from limiting reagent is called theoretical yield = 56.61 grams