Answer:
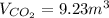
Step-by-step explanation:
Hello,
In this case, the undergoing chemical reaction is:
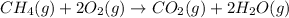
Now, as the stoichiometrical factors are in terms of mole but no information about neither the temperature nor the pressure is given, by means of the Avogadro's law, one could perform the stoichiometric calculations with the given volume as both the pressure and temperature remain the same, that is:
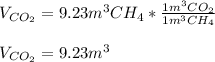
Such 1:1 volume relationship equals the 1:1 molar relationship given in the chemical reaction in terms of their stoichiometric coefficients, therefore, the yielded volume of carbon dioxide is also 9.23m³
Best regards.