Answer: Calcium element will form an ionic bond with carbon.
Step-by-step explanation:
An ionic bond is defined as the bond formed due to transfer of electrons from one atom to another.
Elements which are able to donate their valence electrons are the ones which tend to form an ionic bond. Metals are the species which form ionic bonds when they chemically combine with non-metals.
For example,
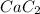 is calcium carbide and in this compound calcium forms an ionic bond with carbon atom by donating its valence electrons.
is calcium carbide and in this compound calcium forms an ionic bond with carbon atom by donating its valence electrons.
A covalent bond is defined as the bond formed due to sharing of electrons between the two elements.
Thus, we can conclude that calcium element will form an ionic bond with carbon.