Answer:
0.016 grams of chloride ion were present in the 0.1000 grams of sample.
Step-by-step explanation:
According to question, 9.00 mL of titrant was added to solution with 0.1000 grams of complex to reach the end point.
Molarity of the silver nitrate solution = 0.0500 M
Volume of the silver nitrate solution = V = 9.00 mL = 0.009 L
1 mL = 1000 L
Moles of silver nitrate = n
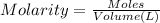
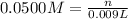
n = 0.00045 mol
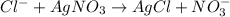
According to 1 mole of silver nitrate reacts with 1 mol of chloride ion, then 0.00045 moles of silver nitrate will :
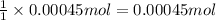 of chloride ions
of chloride ions
Mass of chloride ions :
0.00045 mol × 35.5 g/mol = 0.016 g
0.016 grams of chloride ion were present in the 0.1000 grams of sample.