Answer:
a)
The overall balanced combustion reaction is written as :
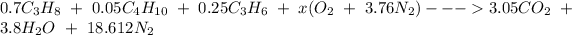
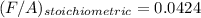
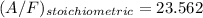
b)
the higher heating values
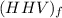 per unit mass of LPG = 49.9876 MJ/kg
per unit mass of LPG = 49.9876 MJ/kg
the lower heating values
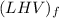 per unit mass of LPG = 46.4933 MJ/kg
per unit mass of LPG = 46.4933 MJ/kg
Step-by-step explanation:
a)
The stoichiometric equation can be expressed as :
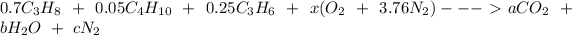
Now, equating the coefficient of carbon; we have:
(0.7×3)+(0.05×4)+(0.25×3) = a
a = 3.05
Also, Equating the coefficient of hydrogen : we have:
(0.7 × 8) +(0.05 × 10) + ( 0.25 × 6) = 2 b
2b = 7.6
b = 3.8
Equating the coefficient of oxygen
2x = 2a + b
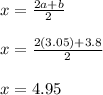
Equating the coefficient of Nitrogen
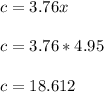
Therefore, The overall balanced combustion reaction can now be written as :
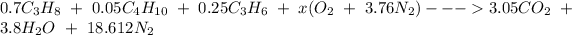
Now; To determine the stoichiometric F/A and A/F ratios; we have:
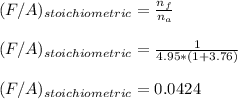
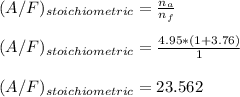
b)
What are the higher and lower heating values per unit mass of LPG?
Let calculate the molecular mass of the fuel in order to determine their mass fraction of the fuel components.
Molecular mass of the fuel
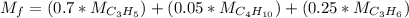
= 30.8 + 2.9 + 10.5
= 44.2 kg/mol
Mass fraction of the fuel components can now be calculated as :
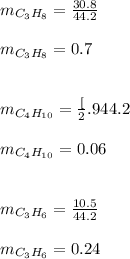
Finally; calculating the higher heating values
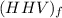 per unit mass of LPG; we have:
per unit mass of LPG; we have:
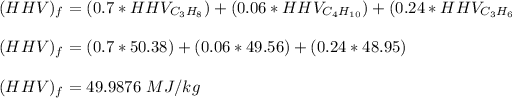
calculating the lower heating values
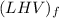 per unit mass of LPG; we have:
per unit mass of LPG; we have:
![(LHV)_f = (HHV)_f - \delta H_w \\ \\ (LHV)_f = (HHV)_f - [(m_w)/(m_f)h_(vap)] \\ \\ (LHV)_f = 49.9876 \ MJ/kg - [(3.8*18)/(44.2)*2.258 \ MJ/kg] \\ \\ (LHV)_f = 46.4933 \ M/kg](https://img.qammunity.org/2021/formulas/chemistry/college/k65szabeyn8583d8v9q9tz71uzza59urt0.png)