Answer:
Molarity of
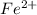 solution is 0.0928 M.
solution is 0.0928 M.
Step-by-step explanation:
Balanced equation:
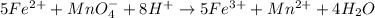
Number of moles of
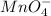 in 10.8 mL of 0.0215 M
in 10.8 mL of 0.0215 M
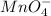 solution
solution
=
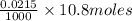 = 0.000232 moles
= 0.000232 moles
Let's assume molarity of
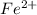 solution is S(M)
solution is S(M)
Number of moles of
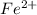 in 12.50 mL of S(M)
in 12.50 mL of S(M)
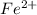 solution
solution
=
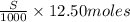 = 0.0125S moles
= 0.0125S moles
According to balanced equation, 5 moles of
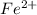 react with 1 mol of
react with 1 mol of
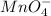
So, 0.0125S moles of
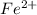 react with
react with
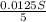 moles of
moles of
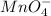
Hence,
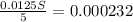
or, S = 0.0928
So, molarity of
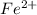 solution is 0.0928 M.
solution is 0.0928 M.