Answer:
The magnitude of the electric force is 0.27N, and the speed of the electron is 211,000,000 m/s, about 0.7c.
Step-by-step explanation:
According to Coulomb's Law, we have that:
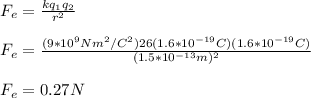
It means that the force of attraction between the nucleus and the electron is 0.27N.
Now, from the definition of centripetal force, we have:
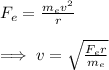
Considering that the mass of an electron is 9.1*10^-31 kg, and we know the magnitude of the electric force and the distance r, we can calculate the speed of the electron:

Having that the speed of light is about 3*10^8 m/s, then the speed of the electron is 0.7c.