The new volume of the soccer ball is 9 litres when temperature inside the can was 25°C and pressure remained constant.
Step-by-step explanation:
Data given:
initial volume of the ball, V1 = 3.6 litres
temperature of the field, initial temperature, T1 = 10°C
temperature in the trash can, final temperature, T2 = 25 °C
pressure =constant throughout
volume of the ball when in trash can, final volume V2 =?
From the data given:
Charles' Law will be applied to know the volume of the ball in the can:
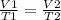
putting the values in the equation after rearranging it:
V2 =
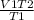
V2 =
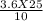
V2 = 9 litres
The volume will be increased to 9 litres when ball will go inside the trash can.