If I have 0.725 moles of gas at a temperature of 105 K and a pressure of 3.75 atmospheres the volume of the gas 1.66 litres.
Step-by-step explanation:
Data given:
number of moles of the gas = 0.725
temperature = 105 K
pressure = 3.75 atm
volume of the gas =?
R = 0.08206 Latm/mole Kelvin
Applying the ideal gas law to calculate the volume of the given gas:
PV = nRT
rearranging the equation to calculate volume:
V =
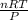
putting the values in the equation:
V =
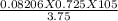
V = 1.66 Litres.
At a temperature of 105 K and pressure of 3.75 atm, 0.725 moles of gas occupy 1.66 litres of volume.