3823.36 kPa will be the pressure be when the temperature reaches 928°C.
Step-by-step explanation:
Data given of aerosol can:
Gas stored at a temperature T1 = 25 °C
pressure of the gas = 103 kPa OR 1.01 atm
If can thrown in fire then temperature T2 = 928 ° C
final pressure when can thrown in fire, P2 =?
Gay Lussac Law is applied to know the final volume:
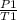 =
=
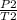
rearranging the equation:
P2 =
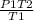
putting the values in the equation:
P2 =
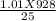
P2 = 37. 49 atm
The pressure will reach 37.49 atm or 3823.36 kPa is the pressure when it is thrown in the fire having temperature of 928 degree celsius.