Complete Question
The complete question is shown on the first uploaded image
Answer:
The value of n is
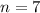
Step-by-step explanation:
From the question we are told that
The value of m = 2
For every value of
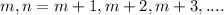
The modified version of Balmer's formula is
![(1)/(\lambda) = R [(1)/(m^2) - (1)/(n^2) ]](https://img.qammunity.org/2021/formulas/physics/college/tus2vkw07noc7oz7oygqfkdinuoobpypaz.png)
The Rydberg constant has a value of
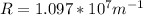
The objective of this solution is to obtain the value of n for which the wavelength of the Balmer series line is smaller than 400nm
For m = 2 and n =3
The wavelength is
![(1)/(\lambda ) = (1.097 * 10^7)[(1)/(2^2) - (1)/(3^2) ]](https://img.qammunity.org/2021/formulas/physics/college/uvesx7dj5gosgnkseyupk8e6tb0c4b0o5r.png)
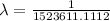
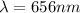
For m = 2 and n = 4
The wavelength is
![(1)/(\lambda ) = (1.097 * 10^7)[(1)/(2^2) - (1)/(4^2) ]](https://img.qammunity.org/2021/formulas/physics/college/znsuzrd71iyp403n5f4xxvx788ep02lq33.png)
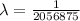
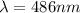
For m = 2 and n = 5
The wavelength is
![(1)/(\lambda ) = (1.097 * 10^7)[(1)/(2^2) - (1)/(5^2) ]](https://img.qammunity.org/2021/formulas/physics/college/xtk1eje64qqpqm4p0ocsk696ajgl0r87m6.png)
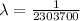
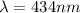
For m = 2 and n = 6
The wavelength is
![(1)/(\lambda ) = (1.097 * 10^7)[(1)/(2^2) - (1)/(6^2) ]](https://img.qammunity.org/2021/formulas/physics/college/bakm9pen0waizrfufoxxpgkf5z2aazpe0h.png)
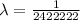
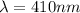
For m = 2 and n = 7
The wavelength is
![(1)/(\lambda ) = (1.097 * 10^7)[(1)/(2^2) - (1)/(7^2) ]](https://img.qammunity.org/2021/formulas/physics/college/dvsxim7ntocrcw7gqgj2nc7315rowohrnb.png)
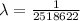
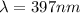
So the value of n is 7