Answer:
c. ΔH° is positive and ΔS° is positive.
Step-by-step explanation:
Hello,
In this case, as the Gibbs free energy for a reaction is defined in terms of the change in the enthalpy and entropy as shown below:
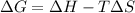
Thus, as the reaction becomes spontaneous (ΔG°<0) at temperatures above 1100K (high temperatures), it necessary that c. ΔH° is positive and ΔS° is positive as the entropy will drive the spontaneousness as it becomes smaller than TΔS°.
Best regards.