Answer:
Work for the gas compression is 93 J.
Step-by-step explanation:
The change in internal internal energy during the whole cycle will be 0 as internal energy is a state function.
According to first law of thermodynamics,
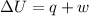
where,
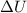 represents change in internal energy, q is the heat exchanged and w is work done.
represents change in internal energy, q is the heat exchanged and w is work done.
Here,
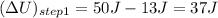 ( q is positive for addition of heat and w is negative for work don by system)
( q is positive for addition of heat and w is negative for work don by system)
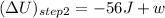 (q is negative for removal of heat)
(q is negative for removal of heat)
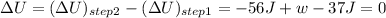
or, w = 93 J