Answer:
20.5 L
Step-by-step explanation:
-The stoichiometric reaction of the 2 gases is:
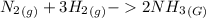
-We apply the combined gas law to determine the resultant volume of the ammonia gas formed:
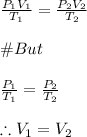
Since, the temperature and pressure remain constant, the volume of the ammonia gas formed is equal to the volume of the reactants.
Hence, the volume after the reaction is 20.5 L