-269.65 °C is the temperature in °C if 1.32 moles of a gas that occupies.35 L at a pressure of 1.06 atm.
Step-by-step explanation:
Data given:
number of moles of the gas, n = 1.32
volume of the gas, V = 0.35 litres
pressure of the gas, P = 1.06 atm
temperature, T =?
R (gas constant) = 0.0821 L atm/mole K
Applying the equation for the ideal gas law:
PV = nRT
rearranging the equation:
T =
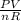
putting the values in the equation:
T =
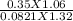
T = 3.5 K
since the unit of temperature obtained is in degrees the temperature in Kelvin is -269.65 degrees. As 0 Kelvin = -273.15 celsius
formula is K -273.15
So,
3.5 - 273.15
= -269.65 celsius