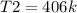Answer:
Step-by-step explanation:
The temperature can be defined as the measurement of the intensity of the heat present in the object. Fahrenheit, kelvin and centigrade are the common scale used for measuring Temperature.
Given:
T1=170C
To convert to Kelvin
= 17+273 =290K
T1 = 290K
Pressure (P)= 95KPa
Specific heat ratio = CP/CV= K
WhereK=1.005/0.718
K = 1.4
The final temperature can be calculated using the formula below.
T2 = CP/CV × T1
=. K × T1
T2 = 1.4 × 290