Answer: The mass of solution the student should use is 256 g
Step-by-step explanation:
Given : 29.3 % w/w which means that 29.3 g of solute is present in 100 g of solution
Thus 100 g of solution contains = 29.3 g of solute
0.50 kg or 500 g of solution contains =
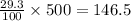 g of solute (1kg=1000g)
g of solute (1kg=1000g)
146.5 g of solute is available in = 500 g of solution
Thus 75 g of solute is available in =
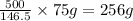 of solution
of solution
Thus the mass of solution the student should use is 256 g