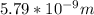According to the information given, the Heisenberg uncertainty principle would be given by the relationship
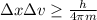
Here,
h = Planck's constant
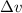 = Uncertainty in velocity of object
= Uncertainty in velocity of object
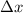 = Uncertainty in position of object
= Uncertainty in position of object
m = Mass of object
Rearranging to find the position
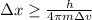
Replacing with our values we have,
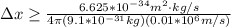
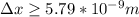
Therefore the uncertainty in position of electron is