Answer:
0.676 grams of manganese (IV) oxide should be added.
Step-by-step explanation:
Moles of chlorine gas = n
Volume of the chlorine gas = V = 205 mL = 0.205 L
Pressure of the chlorine gas = 705 Torr =
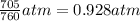
1 atm = 760 Torr
Temperature of the chlorine gas = T = 25°C = 25 + 273 K = 298 K
 ( ideal gs equation)
( ideal gs equation)
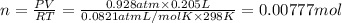
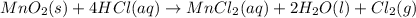
According to reaction, 1 mole of chlorine gas is obtained from 1 mole of manganese(IV) oxide,then 0.00777 moles of chlorine gas will be obtained from :
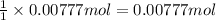 of manganese (IV) oxide
of manganese (IV) oxide
Mass of 0.00777 moles of manganese (IV) oxide:
0.00777 mol × 87 g/mol = 0.676 g
0.676 grams of manganese (IV) oxide should be added.