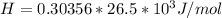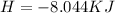Answer:
The heat is
Step-by-step explanation:
From the question we are told that
The pressure is
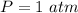
The boiling point is
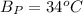
The heat of vaporization at 34°C is =
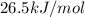
The specific heat of the liquid is
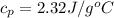
The mass is
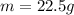
The no of moles of the sample of
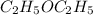 is given as
is given as
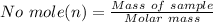
The Molar mass for
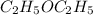 is a value =
is a value =
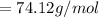
Substituting the value into the above equation
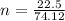
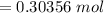
The heat H is mathematically as
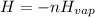
The negative sign show that the heat is for condensing