Answer:
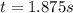
Step-by-step explanation:
Hello,
In this case, for the given reaction, the concentration of dinitrogen oxide is quantified as:
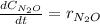
Considering that in molar units (M), the initial and final concentration of dinitrogen oxide are:
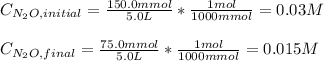
Now, since the rate is zeroth order in dinitrogen oxide, it depends on the rate constant only:
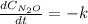
Thus, by integrating from the initial concentration to the final concentration:
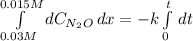
The time finally results:
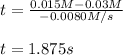
Best regards.