Answer:
5:22 am
Step-by-step explanation:
The gas X decays following a first-order reaction.
The half-life (
 ) is 25 min. We can find the rate constant (k) using the following expression.
) is 25 min. We can find the rate constant (k) using the following expression.
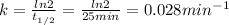
We can find the concentration of X at a certain time (
![[X]](https://img.qammunity.org/2021/formulas/chemistry/college/lus816f6x97mmgynlj1077zsio6ly76e60.png) ) using the following expression.
) using the following expression.
![[X] = [X]_0 * e^(-k * t)](https://img.qammunity.org/2021/formulas/chemistry/college/hcxkqcgvfqo148a2bxu4s3ec2u7dyvxwn9.png)
where,
![[X]_0](https://img.qammunity.org/2021/formulas/chemistry/college/28tpk637njw183rtnaw96aobhrysw7zc7r.png) : initial concentration of X
: initial concentration of X
t: time elapsed
![([X])/([X]_0)= e^(-k * t)\\(1/10[X]_0)/([X]_0)= e^{-0.028min^(-1) * t}\\t=82min](https://img.qammunity.org/2021/formulas/chemistry/college/ifnm2pohyoyiiaycfrha8fbgmwv3gio6pt.png)
The earliest time Professor Utonium can come back to do experiments in the lab is:
4:00 + 82 = 5:22 am