Answer:
Maximum temperature of the water at the bottom of the fall, θ₂ = 16.09°C
Step-by-step explanation:
The height of the waterfall, H = 807 m
At the top of the fall,
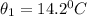
For water, the specific heat capacity, C = 4200 J/kg/°C
Potential energy at the top of the waterfall, PE = mgH
According to the principle of conservation of energy,
Kinetic energy at the bottom of the waterfall = Potential energy at the top
Therefore, KE = mgh
Applying the conservation of energy again,
KE at the bottom = Heat Energy due to raise in temperature
Heat energy due to raise in temperature = MCΔθ
MCΔθ = MgH
CΔθ = gH
Δθ = gH/C
Δθ = (9.81*807)/4200
Δθ = 1.89°C
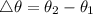
1.89 = θ₂ - 14.2
θ₂ = 14.2 + 1.89
θ₂ = 16.09°C