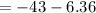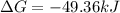Answer:
The reactions free energy
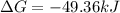
Step-by-step explanation:
From the question we are told that
The pressure of (NO) is
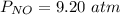
The pressure of (Cl) gas is
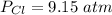
The pressure of nitrosly chloride (NOCl) is
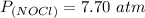
The reaction is
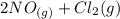 ⇆
⇆
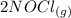
From the reaction we can mathematically evaluate the
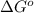 (Standard state free energy ) as
(Standard state free energy ) as
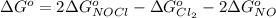
The Standard state free energy for NO is constant with a value
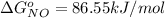
The Standard state free energy for
 is constant with a value
is constant with a value
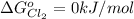
The Standard state free energy for
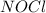 is constant with a value
is constant with a value
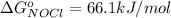
Now substituting this into the equation
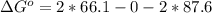
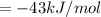
The pressure constant is evaluated as
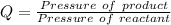
Substituting values
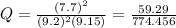
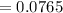
The free energy for this reaction is evaluated as
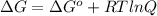
Where R is gas constant with a value of
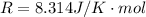
T is temperature in K with a given value of
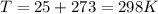
Substituting value
![\Delta G = -43 *10^(3) + 8.314 *298 * ln [0.0765]](https://img.qammunity.org/2021/formulas/chemistry/college/v1a4oic14yn8dvqtp6oynlm5rnzr3dalti.png)