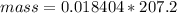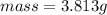Answer:
The mass is
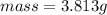
Step-by-step explanation:
From the question we are told that
The current supplied is
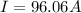
The time taken is t = 37.0 sec
Generally the charge been deposited on the cathode is mathematically represented as
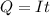
Substituting values
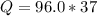
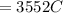
Generally 1 mole of the lead deposited contains
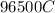
Then
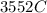 would contain how many moles
would contain how many moles
Not let say x is the number of moles deposited then
Using this mathematical relation
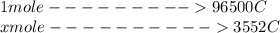
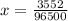
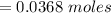
From the the question we are told that lead is been oxidized to lead 2 oxide which implies that the two electron would be accepted by the lead cation
Hence the number of moles that would be deposited at the cathode is
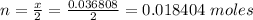
Now the molar mass of lead is constant with a value
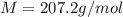
Generally mass is mathematically represented as
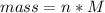
For lead
Substituting values