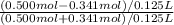Answer:
pH = 6.82
Step-by-step explanation:
To solve this problem we can use the Henderson-Hasselbach equation:
- pH = pKa + log
![([NaOCl])/([HOCl])](https://img.qammunity.org/2021/formulas/chemistry/college/8vdy76sdhj3ic8nm2qt14dgfwdid5bpcqz.png)
We're given all the required data to calculate the original pH of the buffer before 0.341 mol of HCl are added:
- pKa = -log(Ka) = -log(2.9x10⁻⁸) = 7.54
- [HOCl] = [NaOCl] = 0.500 mol / 0.125 L = 4 M
- pH = 7.54 + log
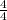
By adding HCl, we simultaneously increase the number of HOCl and decrease NaOCl:
- pH = 7.54 + log
![([NaOCl-HCl])/([HOCl+HCl])](https://img.qammunity.org/2021/formulas/chemistry/college/o0y0nmp2oz4q084yhn3v0nt16cebbyxkoi.png)
- pH = 7.54 + log