Answer:
0.295 L
Step-by-step explanation:
It seems your question lacks the final concentration value. But an internet search tells me this might be the complete question:
" A chemist must dilute 47.2 mL of 150. mM aqueous sodium nitrate solution until the concentration falls to 24.0 mM. He'll do this by adding distilled water to the solution until it reaches a certain final volume. Calculate this final volume, in liters. Be sure your answer has the correct number of significant digits. "
Keep in mind that if your value is different, the answer will be different as well. However the methodology will remain the same.
To solve this problem we can use the formula C₁V₁=C₂V₂
Where the subscript 1 refers to the concentrated solution and the subscript 2 to the diluted one.
- 47.2 mL * 150 mM = 24.0 mM * V₂
And converting into L becomes:
- 295 mL *
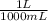 = 0.295 L
= 0.295 L