The calorimeter constant is 816.800 J/degree.
Step-by-step explanation:
Data given:
volume of cold water = 50 ml
initial temperature of the cold water t1 = 19.1 degrees
volume of hot water = 50 ml
temperature of hot water t2 = 54.9 degrees
change in temperature t3= 31.3 degrees
calorimeter constant = ?
energy gained by cold water = energy lost by hot water
q hot water = 50 x 4.184 x (54.9-31.3)
q hot water = 50 x 4.184 x 23.6
= 4937.9 cal/degree
q cold water = 50 x 4.184 x (31.3-19.1)
q cold water = 2552.24 cal/deg
FINAL ENERGY = qhot water - q cold water
final energy = 4937.9 - 2552.24
final energy = 2385.66 cal/deg
calorimeter constant =
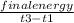
putting the values:
calorimeter constant =
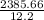
CALORIMETER CONSTANT = 195.22 J/deg
to convert in joules/degree multiply the value with 4.184
= 195.22 x 4.184
= 816.800 J/deg