B. 3.77 L is the new volume occupied by the gas.
Step-by-step explanation:
As per Avogadro's law, which states that if the pressure and temperature held constant, then an equal volume of the gases will occupy an equal number of molecules. It can be written as,
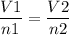
Here, V1, volume of the helium gas = 2.9 L
V2, volume of the additional helium gas in the balloon = ?
n1, moles of helium gas = 0.150 mol
n2, number of moles of additional helium gas = 0.150 + 0.0450 = 0.195 mol
We have to rearrange the equation for V2 as,
V2 =
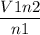
Now Plugin the values as,
V2 =
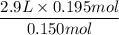
= 3.77 L
So the new volume of the balloon is 3.77 L.