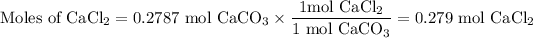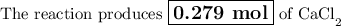Answer:

Step-by-step explanation:
We must do the conversions:
mass of CaCO₃ ⟶ moles of CaCO₃ ⟶ moles of CaCl₂
We will need a chemical equation with masses and molar masses, so, let's gather all the information in one place.
Mᵣ: 100.09 110.98
CaCO₃ + 2HCl ⟶ CaCl₂ + CO₂ + 2HCl
m/g: 27.9
(a) Moles of CaCO₃
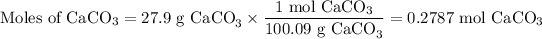
(b) Moles of CaCl₂