7.5 M is the concentration of 60 ml of H3PO4 if it is neutralized by 225 ml of 2 M Ba(OH)2.
Step-by-step explanation:
Data given:
volume of phosphoric acid, Vacid =60 ml
volume of barium hydroxide, Vbase = 225 ml
molarity of barium hydroxide, Mbase = 2M
Molarity of phosphoric acid, Macid =?
the formula for titration is used as:
Macid x Vacid = Mbase x Vbase
rearranging the equation to get Macid
Macid =
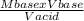
Macid =
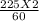
Macid = 7.5 M
the concentration of the phosphoric acid is 7.5 M and the volume is 60 ml. Thus 7.5 M solution of phosphoric acid is used to neutralize the barium hydroxide solution of 2M.