Answer:
4.2 L O₂ is needed to completely react with 2.8 L hydrogen sulfied.
Step-by-step explanation:
Without pressure and temperature we cannot calculate the this vale
We assume that the reaction take place under standard Temperature and Pressure(STP).
At STP, One mole (
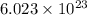 particles) of any gas occupied volume 22.4 L.
particles) of any gas occupied volume 22.4 L.
The balanced equation of this reaction is
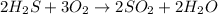
Now we use molar ratio.
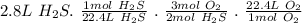
=4.2 L O₂
4.2 L O₂ is needed to completely react with 2.8 L hydrogen sulfied.