Answer and Explanation:
By michaelis mentos equation
Rate of reaction,
![V_0 = (V_(max)[S])/(K_m + [S])](https://img.qammunity.org/2021/formulas/biology/college/7f1c6b4yhp5ipq5qe4lnj0dbu4n9mblkx8.png)
where
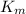 is michaelis mentos constant
is michaelis mentos constant
For same value of Vmax and [S] the rate of reaction will be higher for that as lowest(minimum) value of Km
Here for galactose-6-phosphate the Km value is minimum (Km = 0.02 mm)
So "Galactose-6-phosphate" will best interact with this enzyme and be converted to product