Answer:
-4.278 ×10³ kJ
Step-by-step explanation:
The equation of the reaction is as follows:
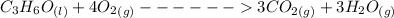
The volume of acetone = 177mL
the density of acetone = 0.788 g/mL
Now;
To determine the mass of acetone ; we use the relation
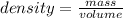
mass = density × volume
mass = 177 mL × 0.788 g/mL
mass = 1.39 × 10² g
Since number of moles =
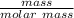
number of moles of acetone =
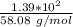
number of moles of acetone = 2.39 moles
Given that -1790 kJ of heat is released for one mole of combustion of acetone. To calculate the amount of heat released with 2.39 moles of acetone; we have:
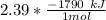
= -4278.1 J
= -4.278 ×10³ kJ