Answer:
It will take 5492 seconds to electroplate 0.5 mm of gold on an object .
Step-by-step explanation:
Mass of gold = m
Volume of gold = v
Surface area on which gold is plated =
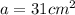
Thickness of the gold plating = h = 0.5 mm = 0.05 cm
1 mm = 0.1 cm
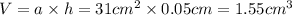
Density of the gold =
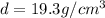
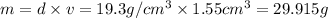
Moles of gold =
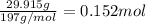
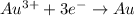
According to reaction, 1 mole of gold required 3 moles of electrons,then 0.152 moles of gold will require :
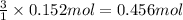 of electrons
of electrons
Number of electrons = N =
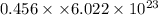
Charge on single electron =
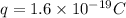
Total charge required = Q
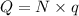
Amount of current passes = I = 8 Ampere
Duration of time = T
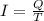
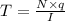
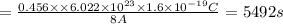
It will take 5492 seconds to electroplate 0.5 mm of gold on an object .