The answer for the following mention bellow.
- Therefore the final temperature of the gas is 260 k
Step-by-step explanation:
Given:
Initial pressure (
 ) = 150.0 kPa
) = 150.0 kPa
Final pressure (
 ) = 210.0 kPa
) = 210.0 kPa
Initial volume (
 ) = 1.75 L
) = 1.75 L
Final volume (
 ) = 1.30 L
) = 1.30 L
Initial temperature (
 ) = -23°C = 250 k
) = -23°C = 250 k
To find:
Final temperature (
 )
)
We know;
According to the ideal gas equation;
P × V = n × R ×T
where;
P represents the pressure of the gas
V represents the volume of the gas
n represents the no of moles of the gas
R represents the universal gas constant
T represents the temperature of the gas
We know;
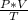 = constant
= constant
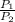 ×
×
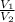 =
=
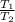
Where;
(
 ) represents the initial pressure of the gas
) represents the initial pressure of the gas
(
 ) represents the final pressure of the gas
) represents the final pressure of the gas
(
 ) represents the initial volume of the gas
) represents the initial volume of the gas
(
 ) represents the final volume of the gas
) represents the final volume of the gas
(
 ) represents the initial temperature of the gas
) represents the initial temperature of the gas
(
 ) represents the final temperature of the gas
) represents the final temperature of the gas
So;
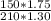 =
=
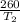
(
 ) =260 k
) =260 k
Therefore the final temperature of the gas is 260 k